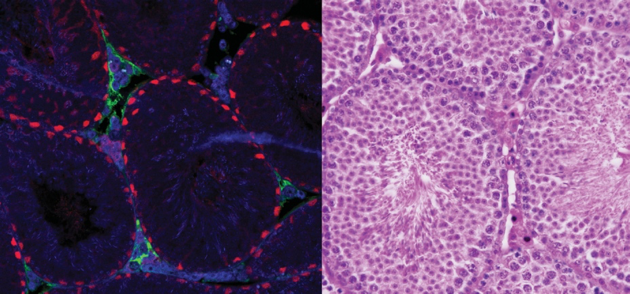
During fetal development, germ cell fate and behaviour is not intrinsically programmed, but instead depends on molecular cues from gonadal somatic cells. In a fetal ovary, germ cells enter meiosis and commit to oogenesis, whereas in a fetal testis, they avoid entry into meiosis and instead undergo mitotic arrest and begin to mature towards spermatogenesis. In mice, these fate decisions occur in the critical window 11.5-13.5 days post coitum (dpc), soon after the germ cells arrive in the nascent gonads.
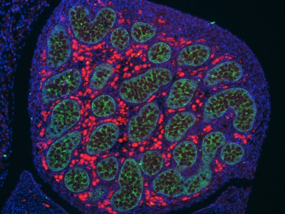 During this critical period of germ cell development, we aim to understand:
During this critical period of germ cell development, we aim to understand:
- How meiosis is triggered in female germ cells
- What makes female germ cells capable of responding to Retionic acid
- How do male germ cells control pluripotency versus differentiation decisions

 During this critical period of germ cell development, we aim to understand:
During this critical period of germ cell development, we aim to understand: