Moritz Group - Developmental programming in disease - Student projects
All projects may be available at both Honours and PhD level.
Periconceptional alcohol exposure: Programming long-term health in offspring
Approximately 80% of Australian women drink alcohol around the time of conception. Whilst many cease or reduce alcohol consumption once pregnancy is confirmed, the long term consequences of exposure around conception are largely unknown. We have developed a pre-clinical rat model of periconceptional alcohol exposure (PCE) and demonstrated that exposure results in fetal growth restriction, impairments in placental development and metabolic, renal and cardiac dysfunction in offspring in adult life. Given that many women drink prior to recognition of pregnancy, the urgent challenge is to understand the mechanisms contributing to disease and design effective intervention strategies.
We currently have projects involving our rat model that are further characterising the impact of PCE on neonatal and long-term offspring health. These include projects involving the development and function of the kidneys (nephron/tubule number and structure; measures of renal function), the heart (vessel morphology/structure; measures of cardiovascular function), the brain and circadian systems (behaviour and circadian rhythms) and the ovary (ovarian reserve, follicle development and fertility).
In addition to describing the offspring phenotypes produced from this periconceptional insult, our lab is currently focused on determining molecular mechanisms underlying the programming of disease outcomes in offspring. We are examining impacts on uterine-embryo interactions at the time of implantation which impact on trophoblast cell invasion and subsequent placental structure. We are also testing the hypothesis that there is altered activity and methylation of the glucocorticoid receptor (GR), which is central to the activity of the hypothalamic-pituitary-adrenal (HPA) axis in both the mother and offspring in response to a stressor. Given that our perturbation is occurring during pregnancy prior to fetal organ and placental formation, epigenetic changes or alterations to maternal physiology are likely mechanisms driving the observed offspring phenotypes.
We are also focusing on testing a safe and feasible intervention during pregnancy. Choline is an essential nutrient and methyl donor and thus can influence DNA methylation. Supplementation with choline has been shown to be safe in human pregnancies. We are currently testing the potential for choline to ameliorate the impacts of PCE placental development and offspring organ function in our preclinical rodent model of periconceptional alcohol exposure.
Co-supervision: Dr David Simmons, Dr Lisa Akison, Prof Vicki Clifton, Dr Oliver Rawashdeh
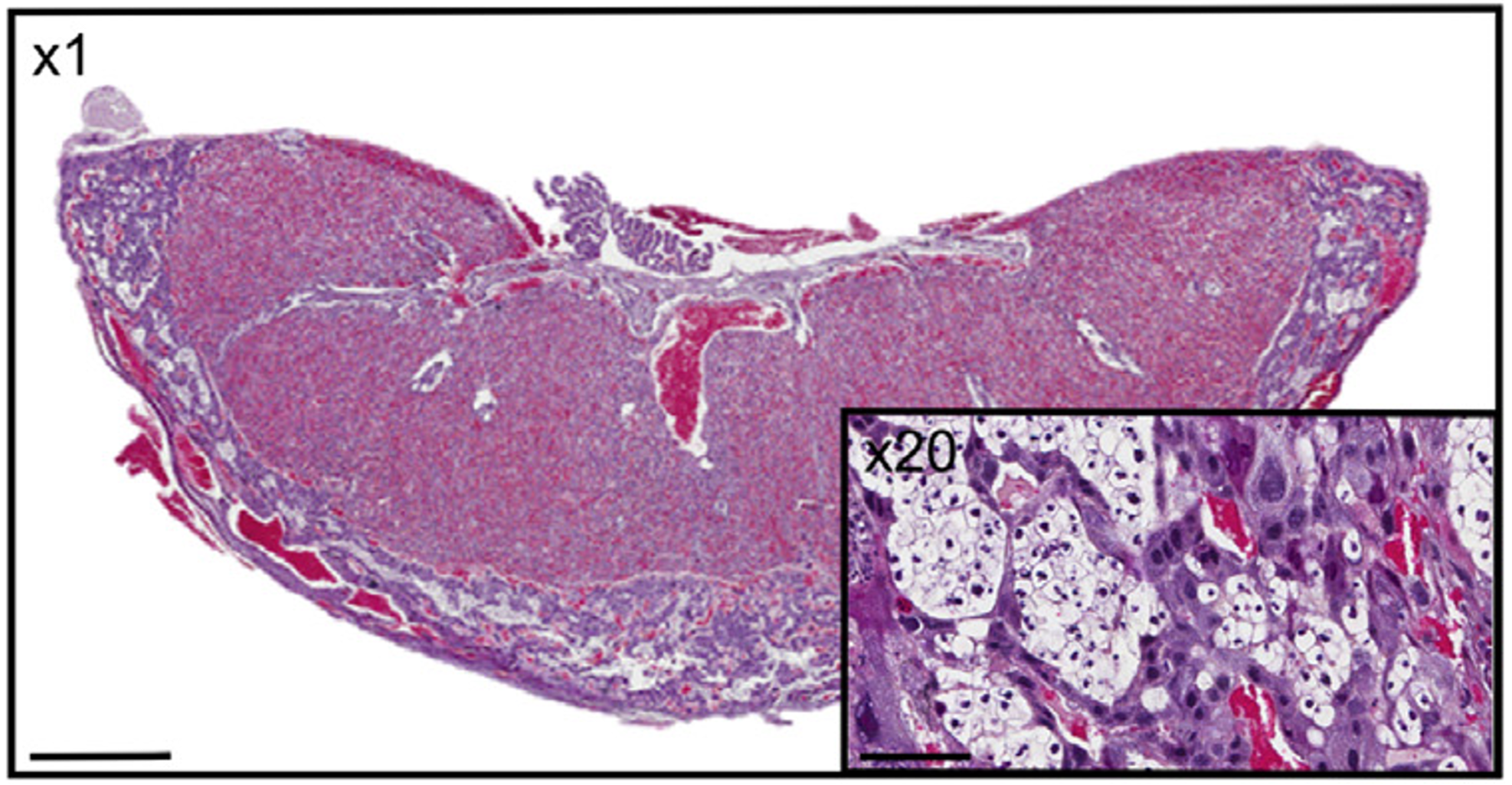
Effect of prenatal insults on the developing placenta
Normal placental function is essential for optimal fetal growth. Thus, many adverse perinatal outcomes including miscarriage, intra-uterine growth restriction (IUGR) and even some fetal defects can be attributed to poor placental function. These can often occur in pregnancies complicated by conditions such as gestational diabetes, asthma/hypoxia, stress or preeclampsia. Our lab, in conjunction with clinical collaborators, is interested in examining placental development, morphology and function in compromised pregnancies.
Transport of glucose from mother to fetus is particularly critical to meet fetal nutrient demands and is controlled by a number of specific placental glucose transporters. In both humans and rodents, glucose can also be stored in the placenta in the form of glycogen, however the function of this glycogen deposition remains a matter of debate. Some evidence suggests placental glycogen provides a source of fuel for the placenta itself, while other studies suggest placental glycogen is stored for later use by the fetus in times of need. While the significance of placental glycogen deposition remains elusive, mounting evidence from both human and animal studies indicates that altered glycogen deposition accompanies many pregnancy complications that adversely affect fetal development. Our lab is interested in determining the circumstances under which glycogen is stored and/or released in the placenta and what role altered placental glycogen deposition may play in inappropriate fetal growth.
Maternal insults during pregnancy also typically result in activation of the hypothalamus-pituitary-adrenal (HPA) axis in both the mother and offspring resulting in elevated glucocorticoids (GC) (cortisol in humans and corticosterone in rodents). GCs exert their action predominantly through binding to the glucocorticoid receptor (GR) which is expressed in many tissues including the placenta. However, activity of the GR is dependent upon the specific isoforms present which in turn is dependent upon DNA methylation of GR tissue-specific promoters. We hypothesise that changes in specific GR isoforms may contribute to altered GR signaling pathways involved in metabolism, growth and inflammation in the placenta.
Co-supervision: Prof Vicki Clifton, Dr Marloes Nitert Dekker, Dr James Cuffe, Dr David Simmons