Noakes Group - Neuromotor control
Peter G. Noakes is investigating the cell and molecular mechanisms that underlie the development and breakdown of the neuro-motor system. Peter’s lab works on the following:
- Cell and molecular mechanisms surrounding the establishment of neuromuscular and motor neuron (CNS) synapses, including electrophysiology, during normal development and in neuromuscular diseases.
- The generation and development of motoneurons in health and in disease (e.g. motor neuron disease {ALS}), using human stem cells to create motor neurons and muscle.
- The role of inflammation in neuromuscular disorders such as motor neuron disease, muscular dystrophy, and myasthenia gravis.
His lab employs biochemistry, immuno-histology, electrophysiology, live cell imaging, behaviour, cell and molecular biology to study these issues. Recent publications on these research areas can be accessed by UQ-eSpace, and PubMed.
The Noakes lab has a number of research projects that are suitable for SCIE, Advanced Science, Honours, MSc (lab Rotations), and PhD students, within the following research areas.
- Motor Neuron Disease (MND)which covers a group of neuromuscular diseases including Amyotrophic Lateral Sclerosis (ALS), and Spinal Muscle Atrophy (SMA). The Noakes lab works with animal models and with human derived stem cells to investigate the cellular and molecular mechanisms of these diseases.We are also embarking upon using AAV and lenti viral vectors to help enhance motor nerve muscle signalling.
- Neuromuscular disorders and inflammation, in this area we are working with chemists that are creating compounds that act to dampen inflammation within muscle and the central nervous system. Our current projects on this centre around Duchenne Muscular Dystrophy, Motor Neuron Disease and Alzheimer's Disease.
- Neuromuscular connections - formation, function and stability in development and disease. This also includes the motor neurons regulation of its excitability and its pre-synaptic connections. Our current projects examine the roles of synaptic adhesion molecules (synaptic laminins and agrin) in the stability of neuromuscular connecitons in health as disease.
- The roles of peri-neuronal nets shaping pre-synaptic inputs onto neurons and their role in regulating neural inflammatory responses in neural degenerative diseases and neural injury.
Visit eSpace for a full list of publications
Phagocytosis of aggrecan-positive perineuronal nets surrounding motor neurons by reactive microglia expressing MMP-9 in TDP-43Q331K ALS model mice. Cheung, Sang Won, Willis, Emily F., Simmons, David G., Bellingham, Mark C., and Noakes, Peter G. (2024). Phagocytosis of aggrecan-positive perineuronal nets surrounding motor neurons by reactive microglia expressing MMP-9 in TDP-43Q331K ALS model mice. Neurobiology of Disease 200 106614 1-13. https://doi.org/10.1016/j.nbd.2024.106614
Prostaglandins in the inflamed central nervous system: potential therapeutic targets. Sheremeta, Chynna-Loren, Yarlagadda, Sai, Smythe, Mark L., and Noakes, Peter G. (2024). Prostaglandins in the inflamed central nervous system: potential therapeutic targets. Current Drug Targets 25. https://doi.org/10.2174/0113894501323980240815113851
Timeline of hypoglossal motor neuron death and intrinsic tongue muscle denervation in high-copy number SOD1G93A mice. Fogarty, Matthew J., Drieberg-Thompson, Joy R., Bellingham, Mark C., and Noakes, Peter G. (2024). Timeline of hypoglossal motor neuron death and intrinsic tongue muscle denervation in high-copy number SOD1G93A mice. Frontiers in Neurology 15 1422943 . https://doi.org/10.3389/fneur.2024.1422943
Cover Image, Volume 50, Issue 3. Cheung, Sang Won, Bhavnani, Ekta, Simmons, David G., Bellingham, Mark C., and Noakes, Peter G. (2024). Cover Image, Volume 50, Issue 3. Neuropathology and Applied Neurobiology 50 (3) . https://doi.org/10.1111/nan.12993
Perineuronal nets are phagocytosed by MMP‐9 expressing microglia and astrocytes in the SOD1G93A ALS mouse model. Cheung, Sang Won, Bhavnani, Ekta, Simmons, David G., Bellingham, Mark C., and Noakes, Peter G. (2024). Perineuronal nets are phagocytosed by MMP‐9 expressing microglia and astrocytes in the SOD1G93A ALS mouse model. Neuropathology and Applied Neurobiology 50 (3) e12982 e12982. https://doi.org/10.1111/nan.12982
Protocol for generating embedding-free brain organoids enriched with oligodendrocytes. Al-mhanawi, Bahaa, Marti, Marta Boira, Morrison, Sean D., Gupta, Pallavi, Alani, Maath, Noakes, Peter G., Wolvetang, Ernst J., and Shaker, Mohammed R. (2023). Protocol for generating embedding-free brain organoids enriched with oligodendrocytes. STAR Protocols 4 (4) 102725 1-18. https://doi.org/10.1016/j.xpro.2023.102725
Chemogenetic modulation of human in vitro motoneuron development accelerates maturation trajectory and electrophysical properties. Morrison, Sean, Shaker, Mohammed, Pietrogrande, Giovanni, Wolvetang, Ernst, and Noakes, Peter (2023). Chemogenetic modulation of human in vitro motoneuron development accelerates maturation trajectory and electrophysical properties. ISN‐ESN 2023 Meeting, Porto, Portugal, 8‐11 August 2023. Chichester, West Sussex, United Kingdom: Wiley-Blackwell
Muscle and its neuromuscular synapse – players in the pathogenesis of motor neuron disease. Noakes, Peter G., Phillips, William D., Jeffree, Rosalind L., Steyn, Frederik J., Wolvetang, Ernst J., Henderson, Rob D., McCombe, Pamela A., and Ngo, Shyuan T. (2023). Muscle and its neuromuscular synapse – players in the pathogenesis of motor neuron disease. Journal of Experimental Neurology 4 (1) 1-5. https://doi.org/10.33696/neurol.4.067
The anaesthetics isoflurane and xenon reverse the synaptotoxic effects of Aβ1–42 on Megf10-dependent astrocytic synapse elimination and spine density in ex vivo hippocampal brain slices. Shi, Dai, Wong, Jaime K. Y., Zhu, Kaichuan, Noakes, Peter G., and Rammes, Gerhard (2023). The anaesthetics isoflurane and xenon reverse the synaptotoxic effects of Aβ1–42 on Megf10-dependent astrocytic synapse elimination and spine density in ex vivo hippocampal brain slices. International Journal of Molecular Sciences 24 (2) 912 1-20. https://doi.org/10.3390/ijms24020912
Investigating the role of GABA in neural development and disease using mice lacking GAD67 or VGAT genes. Bolneo, Erika, Chau, Pak Yan S., Noakes, Peter G., and Bellingham, Mark C. (2022). Investigating the role of GABA in neural development and disease using mice lacking GAD67 or VGAT genes. International Journal of Molecular Sciences 23 (14) 7965 1-18. https://doi.org/10.3390/ijms2314796
Impaired signaling for neuromuscular synaptic maintenance is a feature of Motor Neuron Disease. Ding, Qiao, Kesavan, Kaamini, Lee, Kah Meng, Wimberger, Elyse, Robertson, Thomas, Gill, Melinder, Power, Dominique, Chang, Jeryn, Fard, Atefeh T., Mar, Jessica C., Henderson, Robert D., Heggie, Susan, McCombe, Pamela A., Jeffree, Rosalind L., Colditz, Michael J., Hilliard, Massimo A., Ng, Dominic C. H., Steyn, Frederik J., Phillips, William D., Wolvetang, Ernst J., Ngo, Shyuan T., and Noakes, Peter G. (2022). Impaired signaling for neuromuscular synaptic maintenance is a feature of Motor Neuron Disease. Acta Neuropathologica Communications 10 (1) 61 61. https://doi.org/10.1186/s40478-022-01360-5
Size-dependent dendritic maladaptations of hypoglossal motor neurons in SOD1G93A mice. Fogarty, Matthew J., Mu, Erica W. H., Lavidis, Nickolas A., Noakes, Peter G., and Bellingham, Mark C. (2021). Size-dependent dendritic maladaptations of hypoglossal motor neurons in SOD1G93A mice. Anatomical Record 304 (7) 1562-1581. https://doi.org/10.1002/ar.24542
TDP-43 mutation affects stress granule dynamics in differentiated NSC-34 motoneuron-like cells. Ding, Qiao, Chaplin, Justin, Morris, Matthew J., Hilliard, Massimo A., Wolvetang, Ernst, Ng, Dominic C. H., and Noakes, Peter G. (2021). TDP-43 mutation affects stress granule dynamics in differentiated NSC-34 motoneuron-like cells. Frontiers in Cell and Developmental Biology 9 611601 611601. https://doi.org/10.3389/fcell.2021.611601
Activity-dependent global downscaling of evoked neurotransmitter release across glutamatergic inputs in Drosophila. Karunanithi, Shanker, Lin, Yong Qi, Odierna, G. Lorenzo, Menon, Hareesh, Gonzalez, Juan Mena, Neely, G Gregory, Noakes, Peter G., Lavidis, Nickolas A., Moorhouse, Andrew J., and van Swinderen, Bruno (2020). Activity-dependent global downscaling of evoked neurotransmitter release across glutamatergic inputs in Drosophila. The Journal of Neuroscience 40 (42) 8025-8041. https://doi.org/10.1523/JNEUROSCI.0349-20.2020
Dscam2 suppresses synaptic strength through a PI3K-dependent endosomal pathway. Odierna, G. Lorenzo, Kerwin, Sarah K., Harris, Lucy E., Shin, Grace Ji-eun, Lavidis, Nickolas A., Noakes, Peter G., and Millard, S. Sean (2020). Dscam2 suppresses synaptic strength through a PI3K-dependent endosomal pathway. Journal of Cell Biology 219 (6) e201909143. https://doi.org/10.1083/jcb.201909143
Murine cytomegalovirus infection exacerbates complex IV deficiency in a model of mitochondrial disease. Ferreira, Nicola, Andoniou, Christopher E., Perks, Kara L., Ermer, Judith A., Rudler, Danielle L., Rossetti, Giulia, Periyakaruppiah, Ambika, Wong, Jamie K Y, Rackham, Oliver, Noakes, Peter G., Degli-Esposti, Mariapia A., and Filipovska, Aleksandra (2020). Murine cytomegalovirus infection exacerbates complex IV deficiency in a model of mitochondrial disease. PLoS Genetics 16 (3) e1008604 e1008604. https://doi.org/10.1371/journal.pgen.1008604
Preclinical pharmacokinetics of complement C5a receptor antagonists PMX53 and PMX205 in mice. Kumar, Vinod, Lee, John D., Clark, Richard J., Noakes, Peter G., Taylor, Stephen M., and Woodruff, Trent M. (2020). Preclinical pharmacokinetics of complement C5a receptor antagonists PMX53 and PMX205 in mice. ACS Omega 5 (5) acsomega.9b03735 2345-2354. https://doi.org/10.1021/acsomega.9b03735
Recent research images from the Noakes lab
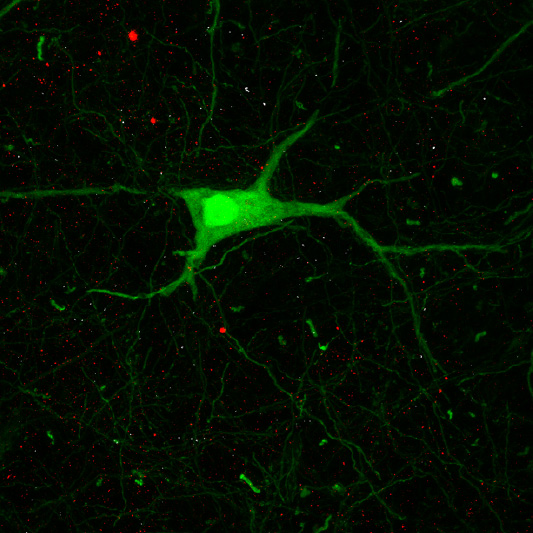
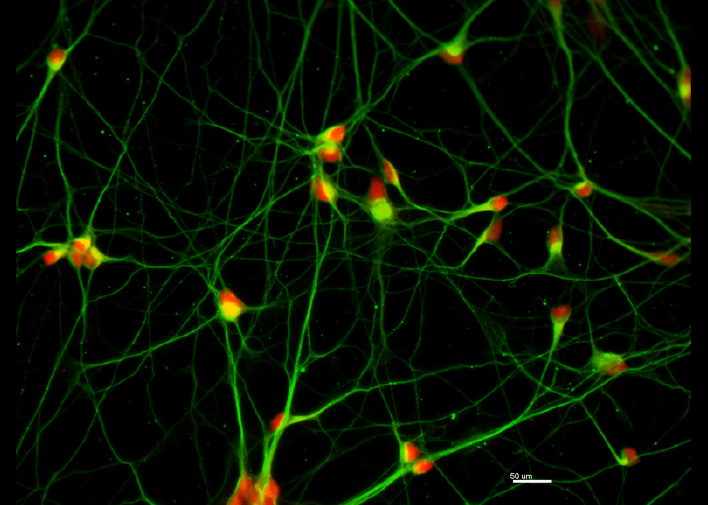
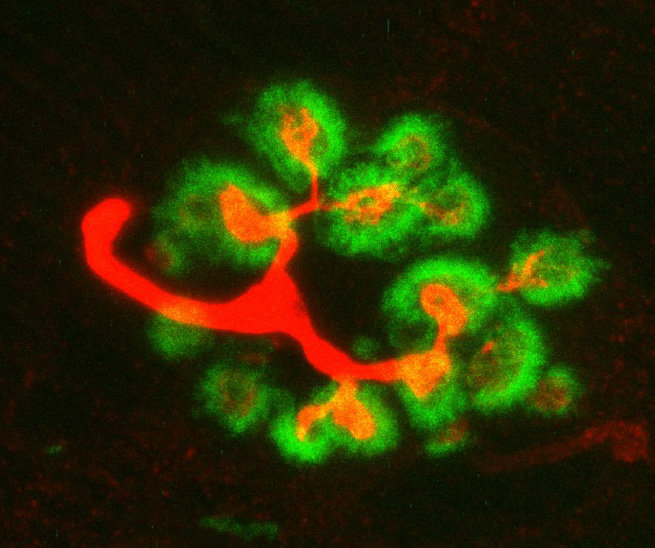
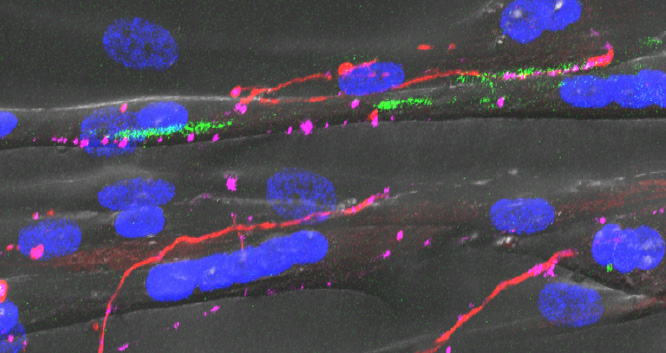
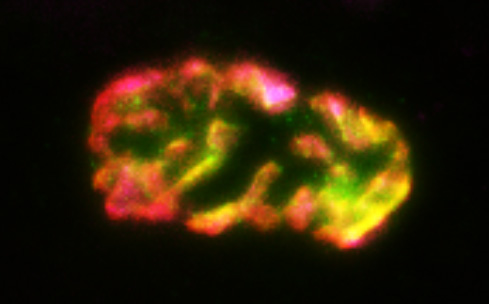
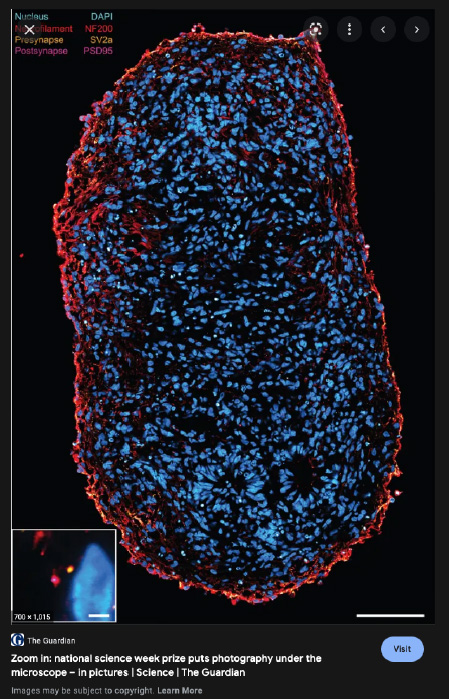
Find out more about our diverse range of research interests.


