Cuffe Group - Placental endocrinology
The aim of our laboratory is to understand the role of the placenta in a range of disease states. The placenta mediates multiple aspects of a successful pregnancy and placental dysfunction is implicated in gestational disorders such as gestational diabetes mellitus, preeclampsia, intrauterine growth restriction, preterm birth and stillbirth. The placenta also regulates many aspects of fetal development such that suboptimal placental function has been shown to increase the risk of metabolic disease, heart disease, kidney disease and psychological disorders in offspring.
Our group utilises both clinically relevant animal models and biological samples provided by pregnant women to better understand how a range of maternal parameters can influence these outcomes. We are particularly interested in understanding how maternal thyroid disease, nutrient status and stress can influence key biological processes necessary for a healthy pregnancy. We are also interested in gaining a better understanding of the impact of certain medications used to treat pregnancy disorders, such as metformin, on maternal and fetal physiology
While not limited to just studying placental endocrinology, the relationship between placental function and hormones in pregnancy is a key theme in multiple projects within the group. This includes hormones of placental origin that may affect the mother and fetus but also maternal hormones that may impact placental function. Our group always characterises outcomes in both males and females separately as fetal sex plays a major role in determining disease risk and severity of symptoms.
The Cuffe group is actively recruiting enthusiastic honours and PhD students who have a desire to undertake research to improve the health of pregnant women and their babies. Please contact James Cuffe directly for further information.
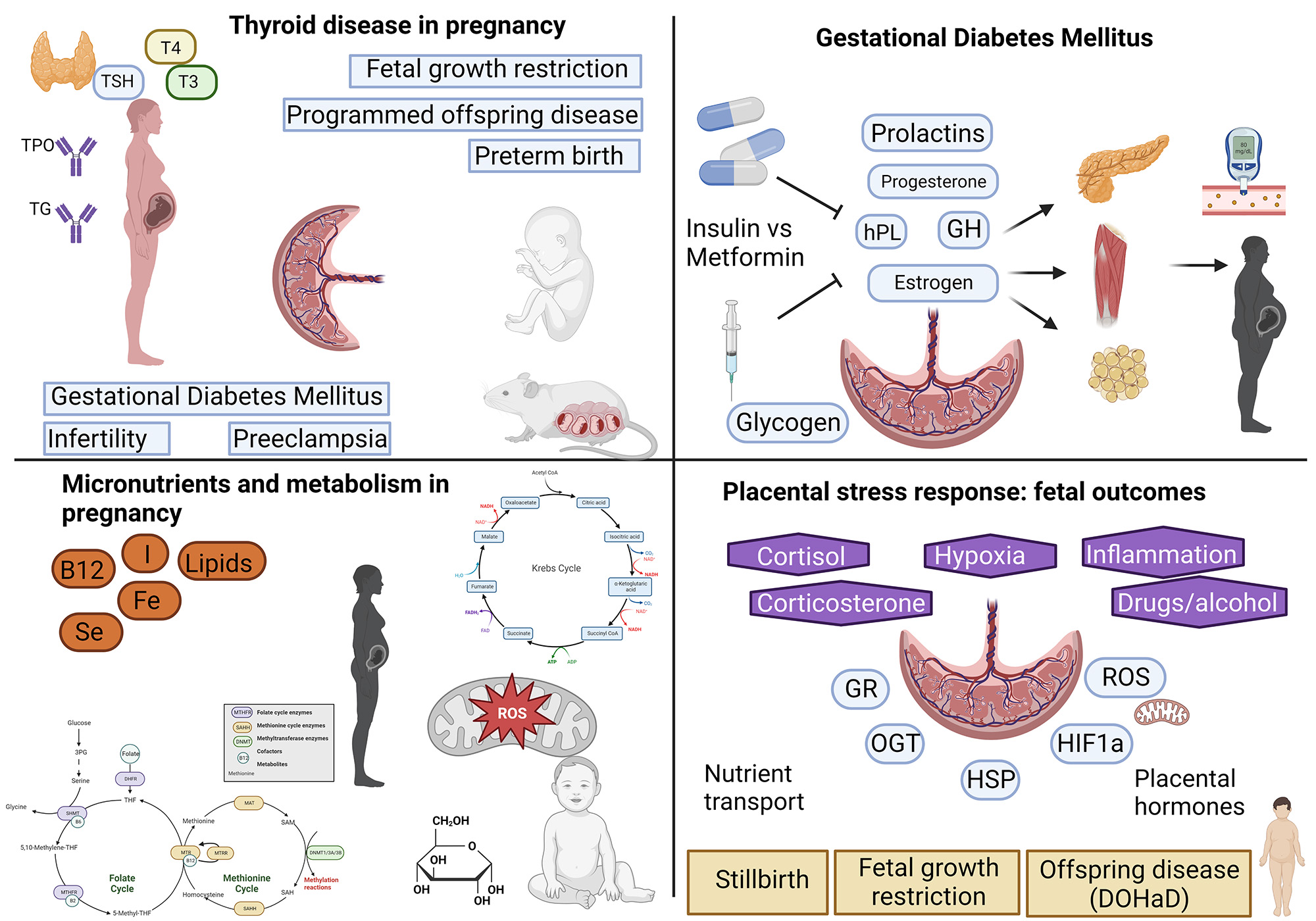
Our laboratory is undertaking a broad range of projects ranging from analysis of dietary deficits in Queensland pregnant women through to characterising the role of specific posttranslational modifications in placental dysfunction. Our research group is highly collaborative and we work with colleagues across Queensland and interstate. Current projects being undertaken in the laboratory include:
- Understanding how metformin treatment for Gestational Diabetes mellitus affects placental function
- Using novel animal models to investigate why women that are deficient in Vitamin B12 are more likely to develop Gestational Diabetes Mellitus
- Investigating risk of pregnancy disorders in relation to micronutrient intake in Queensland women
- Understanding the relationship between thyroid disorders and clinical outcomes in pregnancy
- Using animal models of hypothyroidism and subclinical hypothyroidism to investigate how these common pregnancy disorders contribute to pregnancy complications
- Investigating the role of thyroid antibodies in mediating poor pregnancy outcomes
Our laboratory utilises human samples collected from pregnant women, cell culture experiments and clinically relevant animal models. We are therefore able to ask the same questions using very different approaches which each complementing other aspects of the study.
Specific techniques include:
- Biochemical analysis of various cellular processes- enzymatic assays, colourmetic assays, ELISAs and radioimmunoassays
- Quantification of nutrients in biological samples (preparation for ICPMS and LCMS)
- Quantitative real-time PCR for gene expression analysis, assessment of mitochondrial content and genotyping
- Western blot for protein quantification
- Co-immunoprecipitation to assess interactions between proteins
- Immunohistochemistry and immunofluorescence for protein localisation in tissues
- Histological/pathological staining
- Metabolic analysis such analysis of glucose tolerance (GTT) in rodents and assessment of renal function
- Cardiovascular physiology
- Placental and kidney stereology
NB: All animal experimentation is subject to approval by the University of Queensland Animal Ethics Committee and adheres to the Australian code for the care and use of animals for scientific purposes (National Health and Medical Research Council, 8th Edition, 2013)
Professor Vicki Clifton
Head of Queensland family cohort study and Stillbirth CRE Placenta Working Group
Mater Research, The University of Queensland
Dr David Simmons
School of Biomedical Sciences
The University of Queensland
Dr Marloes Nitert Dekker
School of Chemistry and Molecular Bioscience
The University of Queensland
Dr Helen Barret
Director of Endocrinology
Mater Hospital, QLD
Associate Professor Kerry Richard
Senior Scientist, Conjoint Internal Medicine Laboratory, Pathology Queensland,
School of Biomedical Sciences, Faculty of Health, Queensland University of Technology and
School of Medicine, University of Queensland
Dr Olivia Holland
Lions Medical Research Foundation Fellow
School of Medical Science, Griffith University
School of Biomedical Sciences, Queensland University of Technology
Professor Karen Moritz
Director, Child Health Research Centre and
Professor in School of Biomedical Sciences
The University of Queensland
Professor Anthony Perkins
School of Medical Science
Griffith University
Professor Mary Wlodek
Department of Physiology
The University of Melbourne
View full list of publications on eSpace
Kent NL, Young SL, Akison LK & Cuffe JSM. (2021). Is the link between elevated TSH and gestational diabetes mellitus dependant on diagnostic criteria and thyroid antibody status: a systematic review and meta-analysis. Endocrine.
Hofstee P, James-McAlpine J, McKeating DR, Vanderlelie JJ, Cuffe JSM & Perkins AV. (2021). Low serum selenium in pregnancy is associated with reduced T3 and increased risk of GDM. J Endocrinol 248, 45-57.
Hofstee P, McKeating DR, Bartho LA, Anderson ST, Perkins AV & Cuffe JSM. (2020). Maternal Selenium Deficiency in Mice Alters Offspring Glucose Metabolism and Thyroid Status in a Sexually Dimorphic Manner. 12, 267.
Hofstee P, Bartho LA, McKeating DR, Radenkovic F, McEnroe G, Fisher JJ, Holland OJ, Vanderlelie JJ, Perkins AV & Cuffe JSM. (2019). Maternal selenium deficiency during pregnancy in mice increases thyroid hormone concentrations, alters placental function and reduces fetal growth. J Physiol 597, 5597-5617.
Fisher JJ, McKeating DR, Cuffe JS, Bianco-Miotto T, Holland OJ & Perkins AV. (2019). Proteomic Analysis of Placental Mitochondria Following Trophoblast Differentiation. Front Physiol 10, 1536.
Burgess D, Dorey ES, Gardebjer EM, Bielefeldt-Ohmann H, Moritz K & Cuffe J. (2019). Periconceptional ethanol exposure alters the stress axis in adult female but not male rat offspring. Stress.
Bartho LA, Holland OJ, Moritz KM, Perkins AV & Cuffe JSM. (2019). Maternal corticosterone in the mouse alters oxidative stress markers, antioxidant function and mitochondrial content in placentas of female fetuses. J Physiol 597, 3053-3067.
Hofstee P, McKeating DR, Perkins AV & Cuffe JS. (2018). Placental adaptations to micronutrient dysregulation in the programming of chronic disease. Clin Exp Pharmacol Physiol 45, 871-884.
Holland OJ, Cuffe JSM, Dekker Nitert M, Callaway L, Kwan Cheung KA, Radenkovic F & Perkins AV. (2018). Placental mitochondrial adaptations in preeclampsia associated with progression to term delivery. Cell Death Dis 9, 1150.
Cuffe JSM, Briffa JF, Rosser S, Siebel AL, Romano T, Hryciw DH, Wlodek ME & Moritz KM. (2018). Uteroplacental insufficiency in rats induces renal apoptosis and delays nephrogenesis completion. Acta physiologica 222.

