Projects - Neural stem cell differentiation
Opportunities for new researchers
Our lab uses a variety of techniques to probe neural stem cell biology, including molecular biology, immunocytochemistry, magnetic resonance imaging and mouse behavioural approaches. We also use a range of high-end imaging modalities. Our lab comes from diverse backgrounds, and we are always looking for people with complementary techniques, such as electrophysiology or bioinformatics, to join our group.
Positions are available across our research program for undergraduates, honours students, PhD students, and postdocs, and we are happy to entertain new ideas. If you are interested in joining the lab, please contact Mike.
Understanding the drivers of neural stem cell differentiation
What are the mechanisms that control neural stem cell (NSC) differentiation during embryogenesis, and that enable the generation of the diverse suite of neurons and glia that comprise the brain? This is a key question in developmental neuroscience. My contribution to this field to date has been to reveal central transcriptional regulators that mediate NSC biology within the brain. Using rodent model systems, I demonstrated that transcription factors of the Nuclear Factor One (NFI) family mediate NSC proliferation and differentiation in the embryonic, postnatal and adult nervous system. This work has received international recognition, as evidenced by numerous invited international presentations and high-impact reviews (e.g. Trends in Cell Biology), and forms the framework around which the hypotheses of this program will be addressed.
I am interested in defining how NSC proliferation and differentiation is regulated at a transcriptional and epigenomic level within the developing nervous system. Using the developing mouse brain as a model system, we are using a suite of molecular and cellular techniques to understand how diverse regions of the nervous system are generated, including the cerebral cortex, the cerebellum, the spinal cord and the hypothalamus. For example, within the cerebral cortex, we are investigating how the NFI family of transcription factors mediate NSC differentiation, and how mutations to the NFI family culminate in macrocephaly, and disorders such as Malan syndrome. Moreover, we are using mice lacking the gene Nsd1 (a histone modifying protein) to investigate the development of a human syndrome known as Sotos syndrome, which is also characterised by macrocephaly. In collaboration with Mikael Boden (SCMB), we are also investigating how changes to chromatin landscapes mediate NSC differentiation, and developing bioinformatic tools to enhance the analysis of RNA-seq and ChIP-seq datasets. Collectively, this work will provide fundamental insights into neural development, as well as insights into human neurodevelopmental disorders that arise as a result of abnormal neural stem cell biology in utero.
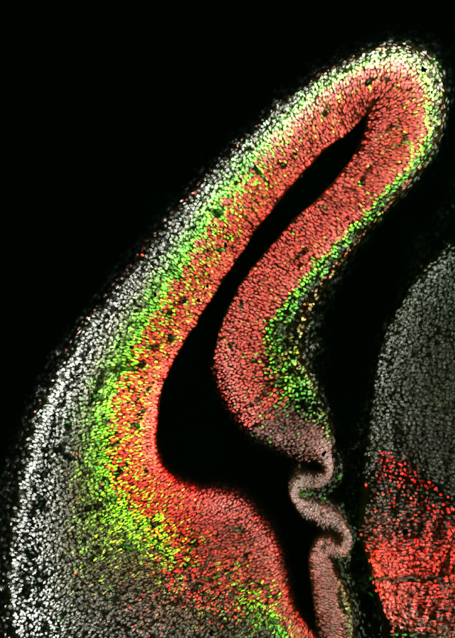
The cerebral cortex is a critical part of the brain. Here, a coronal section of the developing mouse cerebral cortex is shown. There are two main progenitor cell types in the developing cortex, the radial glia (shown in red) and intermediate progenitor cells (shown in green). Defects in the proliferation and differentiation of these cells can give rise to cortical deficits such as microcephaly (smaller brain) or macrocephaly (larger brain). Image courtesy of Lachlan Harris.
Adult neurogenesis
The birth of new neurons within the mature cerebral cortex, a process termed neurogenesis, plays a critical role in learning, memory and spatial navigation. We are investigating various aspects of adult neurogenesis in rodent models, including neural stem cell quiescence and dendrite elongation and spine formation. We are also interrogating the consequences of abnormal neurogenesis using behavioural tests for learning and memory.
We employ a range of transgenic mice to investigate adult neurogenesis, coupled with techniques ranging from immunocytochemistry, behavioural testing, analysis of axonal connectivity and genome-wide sequencing platforms. Given the critical roles that learning and memory play in our everyday lives, and the fact that neurogenesis within the adult brain diminishes with age, this research will provide fundamental insights into how this vital process is co-ordinated at a cellular and molecular level.
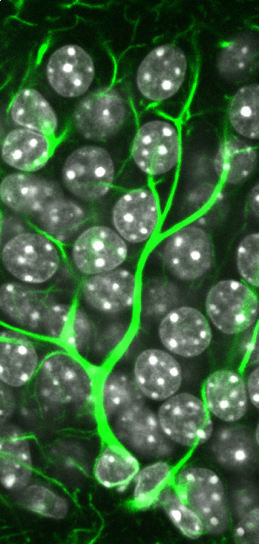
Neural stem cells are found within the dentate gyrus of the adult mammalian brain. Here, a neural stem cell in the dentate gyrus of the adult mouse brain has been identified by the expression of GFAP (green), a marker protein expressed by these cells. Cell nuclei are labelled in white (image courtesy of Lachlan Harris).
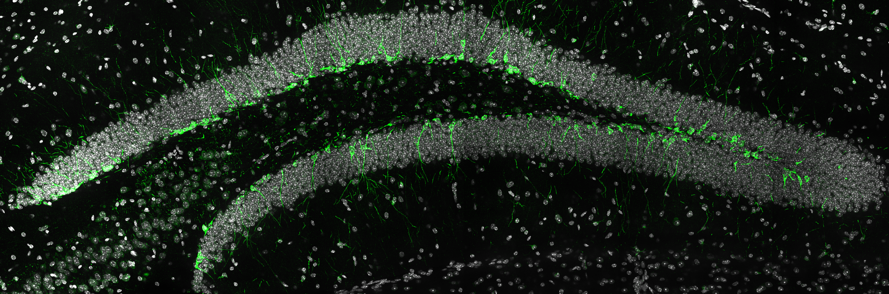
Newly born neurons within the adult dentate gyrus extend processes and become integrated into the existing hippocampal circuitry. In this image, newly born, immature neurons are identified through the expression of DCX (in green) within the adult mouse dentate gyrus. The new neurons can be seen extending processes into the granule cell layer of the dentate gyrus (cell nuclei can be seen in white). Image courtesy of Lachlan Harris.
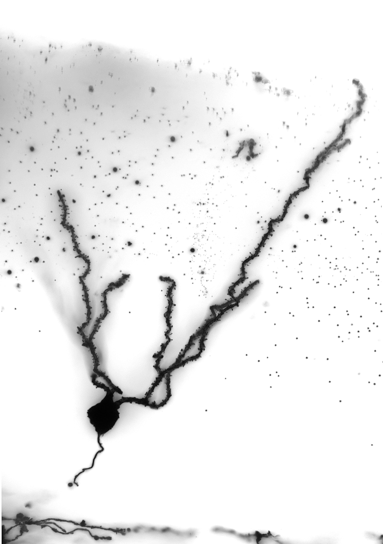
Neurons born within the adult hippocampus from endogenous neural stem cells play a key role in learning and memory. Here, a neuron from the adult mouse dentate gyrus (where neurogenesis within the adult hippocampus takes place) is labelled. The elaborate dendritic tree of the neuron is critical to enable learning and memory to take place. Image courtesy of Sabrina Oishi.
Identifying how abnormal neural stem cell biology contributes to disease
The importance of NSC biology to brain development is underscored by disorders associated with abnormal NSC differentiation, including autism, hydrocephalus, microcephaly and macrocephaly. Despite the role of aberrant NSC development to these disorders, our understanding of the cellular and molecular deficits that contribute to disease onset and progression remains limited. Recently, my work has begun to focus on these disorders. Moreover, as the transcriptional landscape of many cancers resembles that of stem cells during development, I am also applying my expertise to understand how abnormal transcriptional activity contributes to cancer progression. This approach has gained significant traction, as evidenced by international awards (2015 Innovator Award, Hydrocephalus Association) and grants (Simons Foundation Autism Research Initiative, 2018-2019, 2023-2024; Cancer Council Queensland, 2016-2017) I have received.
We are using our fundamental understanding of NSC biology to investigate numerous neurodevelopmental disorders. We are investigating macrocephaly (enlarged brain size), with a specific focus on the overgrowth syndromes Malan syndrome, Sotos syndrome and Luscan-Lumish syndrome. This work is supported by clinical collaborators and is being performed in collaboration with patient advocacy groups including the Malan Syndrome Foundation and CombinedBrain.
