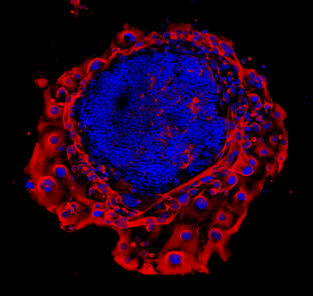
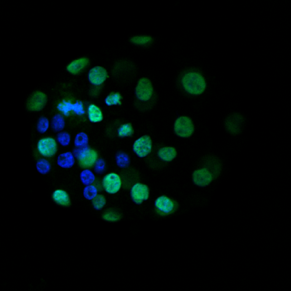
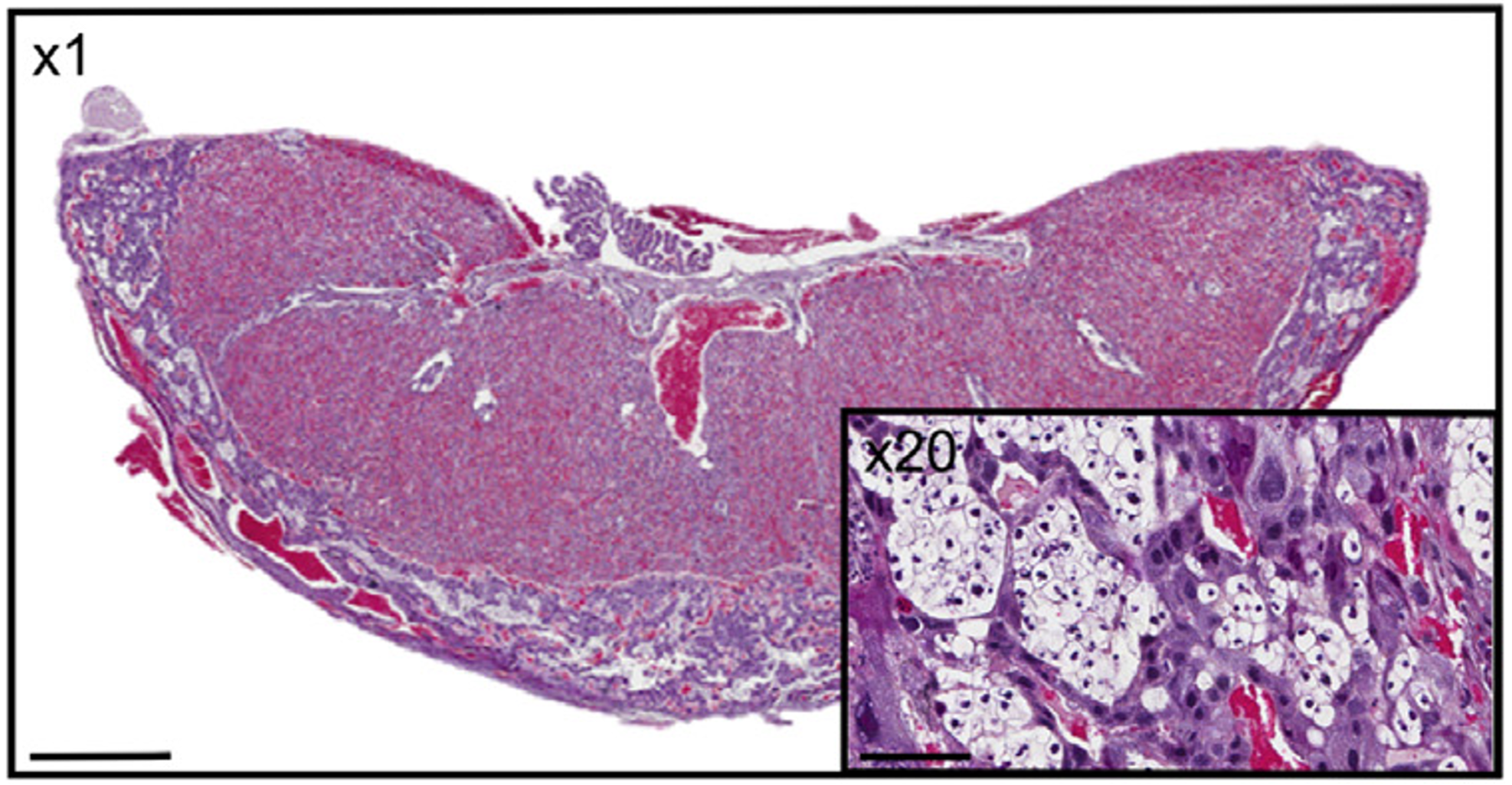
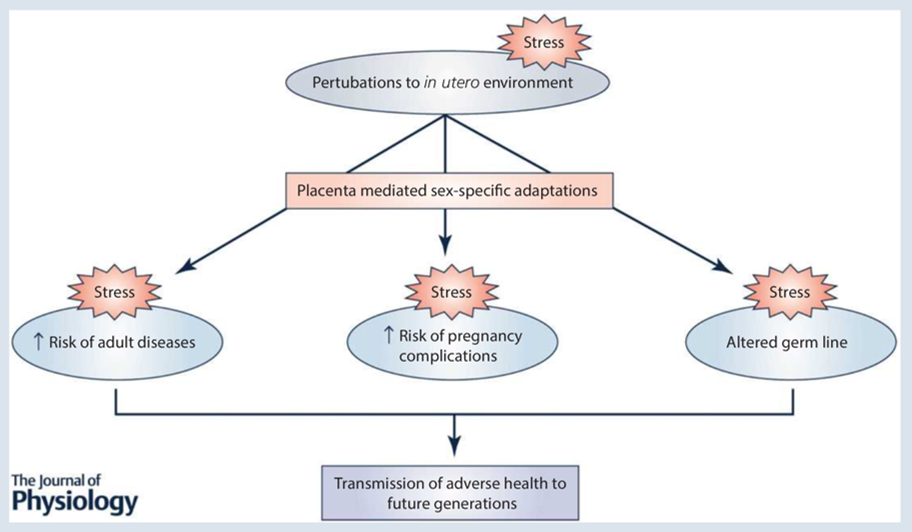
The aim of our laboratory is to understand how prenatal perturbations contribute to an increased risk of developing adult-onset disease in offspring. This research area provides evidence for a now well-known hypothesis known as the Developmental Origins of Health and Disease (DOHaD). By identifying organs and systems that are affected by prenatal insults, this provides us with a chance to intervene and prevent the burden of many chronic diseases in the Australian population. Our lab uses preclinical models that mimic common prenatal insults, such as stress and alcohol exposure, and conducts detailed analyses of offspring development and adult offspring metabolic, cardiovascular, renal, reproductive and neurological/behavioural function. Specific organs of interest include the kidney, placenta, heart, brain and ovary. We also have clinical projects available with collaborators at the Mater Research Institute/Translational Research Institute and the Child Health Research Centre that specifically look at the implications of prenatal alcohol during pregnancy on placental morphology/function and offspring health.
Dr Natasha Reid
Child Health Research Centre
The University of Queensland
Dr James Cuffe
School of Biomedical Sciences
The University of Queensland
Dr David Simmons
School of Biomedical Sciences
Mater Medical Research Institute
Translational Research Institute
The University of Queensland
Dr Marloes Nitert Dekker
School of Chemistry and Molecular Biosciences
Centre for Clinical Research
The University of Queensland
Dr Oliver Raweshdeh
School of Biomedical Sciences
The University of Queensland
Professor Vicki Clifton
Mater Medical Research Institute
Translational Research Institute
The University of Queensland
Professor Mary Wlodek
Department of Physiology
The University of Melbourne
Find out more about our diverse range of research interests.