My lab is currently focusing on the reasons why cholinergic neurons of the basal forebrain die in Alzheimer’s disease, what contribution their loss makes to cognitive decline and whether manipulating neurotrophic signalling (NGF, BDNF, TrkA/B, p75) can protect or restore cognitive function, and what role the neurotrophins play in the normal function of these neurons.
The lab is supported by:
Australia Research Council Discovery Project
National Health and Medical Research Council Centres of Research Excellence
For a full list of publications, visit eSpace
- Qian, Lei, Rawashdeh, Oliver, Kasas, Lei, Milne, Michael R., Garner, Nicholas, Sankorrakul, Kornraviya, Marks, Nicola, Dean, Matthew W., Kim, Pu Reum, Sharma, Aanchal, Bellingham, Mark C. & Coulson, Elizabeth J. (2022) Cholinergic basal forebrain degeneration due to sleep-disordered breathing exacerbates pathology in a mouse model of Alzheimer’s disease. Nature Communications 13, 6543 (2022). https://doi.org/10.1038/s41467-022-33624-y
- Pearson, Ella, Siskind, Dan, Hubbard, Ruth, Gordon, Emily, Coulson, Elizabeth, Arnautovska, Urska and Warren, Nicola (2022). Frailty and Treatment-Resistant Schizophrenia: A Retrospective Cohort Study. Community Mental Health Journal. doi: 10.1007/s10597-022-00998-8
- Zhou, Xiaoqing Alice, Ngiam, Grace, Qian, Lei, Sankorrakul, Kornraviya, Coulson, Elizabeth J. and Chuang, Kai-Hsiang (2022). The basal forebrain volume reduction detected by MRI does not necessarily link with the cholinergic neuronal loss in the Alzheimer's Disease mouse model. Neurobiology of Aging, 117, 24-32. doi: 10.1016/j.neurobiolaging.2022.03.017
- Conroy, Jacinta N. and Coulson, Elizabeth J. (2022). High-affinity TrkA and p75 neurotrophin receptor complexes: a twisted affair. Journal of Biological Chemistry, 298 (3) 101568, 101568. doi: 10.1016/j.jbc.2022.101568
- Sankorrakul, Kornraviya, Qian, Lei, Thangnipon, Wipawan and Coulson, Elizabeth J (2021). Is there a role for the p75 neurotrophin receptor in mediating degeneration during oxidative stress and after hypoxia?. Journal of Neurochemistry, 158 (6) jnc.15451, 1-15. doi: 10.1111/jnc.15451
- Groves, Natalie, O’Keeffe, Imogen, Lee, Wendy, Toft, Alexandra, Blackmore, Daniel, Bandhavkar, Saurabh, Coulson, Elizabeth J., Bartlett, Perry F. and Jhaveri, Dhanisha J. (2020). Blockade of TrkB but not p75 NTR activates a subpopulation of quiescent neural precursor cells and enhances neurogenesis in the adult mouse hippocampus. Developmental Neurobiology, 79 (9-10) dneu.22729, 868-879. doi: 10.1002/dneu.22729
- Meier, Sonja, Alfonsi, Fabienne, Kurniawan, Nyoman D., Milne, Michael R., Kasherman, Maria A., Delogu, Alessio, Piper, Michael and Coulson, Elizabeth J. (2019) The p75 neurotrophin receptor is required for the survival of neuronal progenitors and normal formation of the basal forebrain, striatum, thalamus and neocortex. Development, dev.181933. doi:10.1242/dev.181933
- Martínez-Mármol R, Mohannak N, Qian L, Wang T, Gormal RS, Ruitenberg MJ, Vanhaesebroeck B, Coulson EJ and Meunier FA. (2019) p110δ PI 3-kinase inhibition perturbs APP and TNFα trafficking, reduces plaque burden, dampens neuroinflammation and prevents cognitive decline in an Alzheimer's disease mouse model. Journal of Neuroscience 30 July 2019, 0674-19; DOI: https://doi.org/10.1523/JNEUROSCI.0674-19.2019
- Milne MR, Qian L, Turnbull MT, Kinna G, Collins BM, Teasdale RD, Coulson EJ. (2019) Downregulation of SNX27 expression does not exacerbate amyloidogenesis in the APP/PS1 Alzheimer's disease mouse model. Neurobiol Aging. 2019 May;77:144-153. doi: 10.1016/j.neurobiolaging.2019.01.011. Epub 2019 Jan 25.
- Boskovic Z, Meier S, Wang Y, Milne MR, Onraet T, Tedoldi A, Coulson EJ. (2019) Regulation of cholinergic basal forebrain development, connectivity, and function by neurotrophin receptors. Neuronal Signaling, Feb 04, 2019, 3 (1); DOI: 10.1042/NS20180066
- Lee HL, Li Z, Coulson EJ, Chuang KH. (2019) Ultrafast fMRI of the rodent brain using simultaneous multi-slice EPI. Neuroimage. 2019 Jul 15;195:48-58. doi: 10.1016/j.neuroimage.2019.03.045. Epub 2019 Mar 22.
- Qian L, Milne MR, Shepheard S, Rogers ML, Medeiros R, Coulson EJ. (2018) Removal of p75 Neurotrophin Receptor Expression from Cholinergic Basal Forebrain Neurons Reduces Amyloid-β Plaque Deposition and Cognitive Impairment in Aged APP/PS1 Mice. Mol Neurobiol. 2018 Oct 29. doi: 10.1007/s12035-018-1404-2. [Epub ahead of print]
- Boskovic Z, Milne MR, Qian L, Clifton HD, McGovern AE, Turnbull MT, Mazzone SB & Coulson EJ. (2018) Cholinergic basal forebrain neurons regulate fear extinction consolidation through p75 neurotrophin receptor signalling. Translation psychiatry 8 (1): 199. 21 September 2018 doi: 10.1038/s41398-018-0248-x.
- Turnbull MT, Boskovic Z, Coulson EJ. (2018) Acute Down-regulation of BDNF Signaling Does Not Replicate Exacerbated Amyloid-β Levels and Cognitive Impairment Induced by Cholinergic Basal Forebrain Lesion. Frontiers in Molecular Neuroscience 11: 51. 22 February 2018 doi.org/10.3389/fnmol.2018.00051
- May, Linda M., Anggono, Victor, Gooch, Helen M., Jang, Se E., Matusica, Dusan, Kerbler, Georg M., Meunier, Frederic A., Sah, Pankaj and Coulson, Elizabeth J. (2017) G-protein-coupled inwardly rectifying potassium (GIRK) channel activation by the p75 neurotrophin receptor is required for amyloid beta toxicity. Frontiers in Neuroscience, 11 455: . doi:10.3389/fnins.2017.00455
- Coulson, Elizabeth J. and Bartlett, Perry F. (2017) An exercise path to preventing Alzheimer's disease : An Editorial Highlight on 'Exercise and BDNF reduce Ab production by enhancing alpha-secretase processing of APP'. Journal of Neurochemistry, 142 2: 191-193. doi:10.1111/jnc.14038
- Turnbull, Marion T. and Coulson, Elizabeth J. (2017) Cholinergic basal forebrain lesion decreases neurotrophin signaling without affecting tau hyperphosphorylation in genetically susceptible mice. Journal of Alzheimer's Disease,55 3: 1141-1154. doi:10.3233/JAD-160805
- Matusica, D., Alfonsi, F., Turner, B. J., Butler, T. J., Shepheard, S. R., Rogers, M. L., Skeldal, S., Underwood, CK., Mangelsdorf, M & Coulson, E. J. (2016). Inhibition of motor neuron death in vitro and in vivo by a p75 neurotrophin receptor intracellular domain fragment. J Cell Sci, 129(3), 517-530.
- Kerbler, G. M., Nedelska, Z., Fripp, J., Laczo, J., Vyhnalek, M., Lisy, J., Hamlin, AS., Rose, S., Hort J. & Coulson, E. J. (2015). Basal Forebrain Atrophy Contributes to Allocentric Navigation Impairment in Alzheimer's Disease Patients. Front Aging Neurosci, 7, 185.
- Kerbler, G. M., Fripp, J., Rowe, C. C., Villemagne, V. L., Salvado, O., Rose, S. & Coulson, EJ. Alzheimer's Disease Neuroimaging, I. (2015). Basal forebrain atrophy correlates with amyloid beta burden in Alzheimer's disease. Neuroimage Clin, 7, 105-113.
- Coulson, E. J., & Andersen, O. M. (2015). The A-B-C for SORting APP. J Neurochem, 135(1), 1-3.
- Matusica, D., & Coulson, E. J. (2014). Local versus long-range neurotrophin receptor signalling: endosomes are not just carriers for axonal transport. Semin Cell Dev Biol, 31, 57-63.
- Edwards, S. R., Hamlin, A. S., Marks, N., Coulson, E. J., & Smith, M. T. (2014). Comparative studies using the Morris water maze to assess spatial memory deficits in two transgenic mouse models of Alzheimer's disease. Clin Exp Pharmacol Physiol, 41(10), 798-806. doi: 10.1111/1440-1681.12277
- Boskovic, Z., Alfonsi, F., Rumballe, B. A., Fonseka, S., Windels, F., & Coulson, E. J. (2014). The role of p75NTR in cholinergic basal forebrain structure and function. J Neurosci, 34(39), 13033-13038. doi: 10.1523/JNEUROSCI.2364-14.2014
- D Matusica, S Skeldal, AM Sykes, N Palstra, A Sharma, EJ Coulson (2013) An intracellular domain fragment of the p75 neurotrophin receptor enhances TrkA receptor function. Journal of Biological Chemistry 288:11144-54.
We are currently unable to recruit SCIE, Honours or Masters students.
If you are interested in a HDR project with Professor Coulson, you will need to apply through the University of Melbourne. Details to follow.
Current grants
Understanding glymphatic contribution to cognitive impairment (2023–2025) Dementia Australia Research Foundation Project Grants (Dr Zengmin Li)
Decoding the brain network of memory formation (2023-2028) ARC-Discovery Projects (Associate Professor Kai-Hsiang Chuang & Professor Dr Christophe Bernard, Aix-Marseille University, France)
- Centre of Research Excellence in Mechanisms In NeuroDegeneration - Alzheimer's Disease (MIND-AD CRE) (2024-2029) NHMRC Centres of Research Excellence
- Professor Anders Nykjaer - Aarhus University, Denmark
- Associate Professor Jakob Hort - Charles University, Prague, Czech Republic
- Associate Professor Stephen Rose - UQCCR
- Professor Pankaj Sah - QBI
- Professor Fred Meunier - QBI
- Associate Professor Kai-Hsiang Chuang – QBI
- Dr Eamonn Eeles, Geriatrician & Dementia Researcher, Prince Charles Hospital
- Dr Oliver Rawashdeh - School of Biomedical Sciences
Sleep apnea study
Professor Coulson’s team are researching what factors makes sleep disruption a risk factor for Alzheimer's disease. They are beginning a study that will follow patients aged 55 to 75 with sleep apnoea over an extended period, to determine whether using a continuous positive airway pressure (CPAP) ventilator, which keeps airways open during sleep, protects against brain degeneration and lowers the risk of dementia.
Sterling's Dream
Professor Coulson and colleagues, Dr Eamon Eeles (The Prince Charles Hospital) and Professor Stephen Rose (CSIRO), are involved in research studying what factors make the gold standard drugs for Alzheimer's disease more likely to be effective. Information on this study (Sterling’s Dream) can be found here.
Obstructive sleep apnea (OSA) is the most common type of sleep disruption. It affects more than 50% of the elderly adult population and occurs due to the collapse of the upper airways, particularly during rapid eye movement (REM) sleep and it is usually associated with a reduction in blood oxygen saturation. OSA is a strong epidemiological risk factor for the development of dementia, but also cardiovascular disease and diabetes.
We have developed a naturalistic mouse model of OSA through selectively ablating cholinergic neurons in the brainstem with injection of a ribosomal inactivating saporin-conjugated urotensin-2 peptide (UII-SAP) in the brain. The mice replicate key features of human OSA: altered breathing during sleep, sleep disruption, moderate intermittent hypoxemia and cognitive impairment. When we induced OSA in a familial AD model, the mice displayed exacerbation of cognitive impairment and pathological features of AD, including increased levels of amyloid-beta (Aβ) and inflammatory markers, as well as selective degeneration of cholinergic basal forebrain neurons. We also revealed that the above neurodegenerative symptoms could be prevented by inhibition of neural cell death receptor p75 neurotrophin receptor (p75NTR) and/or hypoxia inducible factor 1 alpha activity (HIF1α).
It is the first naturalistic OSA mouse model in the field which otherwise uses fluxing oxygen concentrations in piped air requiring expensive hypoxia chambers and gas mixtures. Our OSA mouse display the above symptoms from two weeks after surgery, and mice can be group housed in standard cages with assessments performed at any time without disrupting OSA conditions. The model can be used to investigate the effects of sleep apnea including in vivo testing of drugs in development for a variety of chronic conditions.
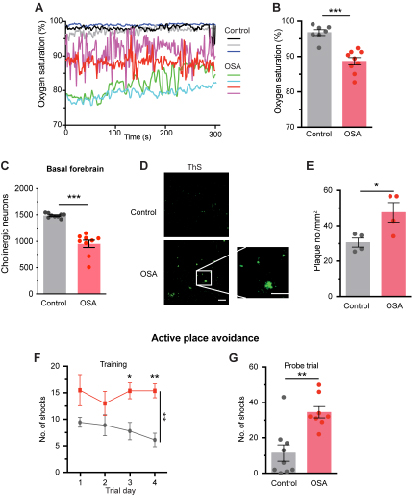
Obstructive sleep apnea (OSA) model
The Obstructive sleep apnea (OSA) mouse has altered breathing pattern and induces hypoxia.
The blood oxygen saturation is significantly reduced during the sleep period in OSA mice (A and B).
Obstructive sleep apnea exacerbates major AD hallmarks in the APP/PS1 mice.
The OSA APP/PS1 mice had lesions of cholinergic basal forebrain (C) and significantly increased levels of thioflavin S-positive Aβ plaques (D and E) in the cortex.
Obstructive sleep apnea exacerbates cognitive impairment in the APP/PS1 model.
The OSA APP/PS1 mice received significantly more shocks on the last two training days (F) and during the probe trial of the active place avoidance test (G).
Find out more about our diverse range of research interests.