Adeno-associated virus (AAV)
About AAV
The UQ Viral Vector Core offers an Adeno-Associated Virus (AAV) particle production and consulting service utilising optimised protocols and workflows. This service generates ready-to-use AAV particle concentrates suitable for in vitro or in vivo applications, with typical titres of ≥ 1011 viral genome copies (vgc) per ml or 5x1012-1013 vgc/mL, respectively.
AAV biology and production
AAVs are helper-dependent ssDNA parvoviruses, meaning they require components from other viruses to complete their life cycle. Wild-type AAVs contain two open reading frames (Rep and Cap), flanked by two inverted terminal repeats (ITRs). The ITRs contain recognition sites for initiation and termination of replication, and are the only cis-acting elements required for vector production. For research purposes, the Rep and Cap genes (encoding proteins for replication and capsid production) are replaced with a user-defined cassette containing desired genes of interest. The Rep and Cap genes are supplied in trans during vector production, which ensures the virus is replication-deficient once removed from the production system.
In addition, AAVs require 'helper' genes (E4, E2A and VA) from a co-infecting virus (usually Adenovirus), which are supplied in a separate plasmid. Typically, the Rep/Cap, Helper and Transfer plasmid (containing the user-defined cassette) are transfected into HEK 293 cells containing the Adenovirus E1+ gene, promoting AAV production. After a few days, the AAV particles are collected from the cells/supernatant and are purified into a concentrated solution that is ready to use.
Wild-type AAVs integrate into the host genome at a specific site on human chromosome 19, however recombinant AAVs (for research purposes) rarely integrate, as they lack the Rep gene. Instead, the AAV genome remains largely episomal, where it can persist long-term in cells that do not divide. As AAVs contained single-stranded DNA, they rely on the host's machinery to synthesise the complementary DNA strand, which can limit transduction efficiency. If rapid expression is required, users may prefer to use a self-complementary system whereby the sequences spontaneously self-anneal. This eliminates the need for second-strand synthesis, however it also halves the packaging capacity (<2.4 kb), so can only be used for small constructs.
Why choose AAV?
AAVs are popular gene delivery tools for both research and clinical applications due to a number of reasons, including their ability to target specific tissues in vivo; low immunogenicity; and stable, long-term gene expression. Recombinant AAVs lack the genes required for genome integration and viral replication, resulting in primarily episomal activity (genome integration is extremely rare). Therefore, AAVs are most beneficial when targeting non-dividing cells (e.g. cardiomyocytes and neurons), where they can persist for long periods of time. They are, however, limited by their packaging capacity of ~4.7 kb which has lead to the development of more complex dual-AAV split vectors (with generally lower success than single vectors).
| Advantages | Disadvantages |
|---|---|
|
|
AAV capsids currently available
The viral capsid serotype determines the tissue specificity of AAV vectors. This selection is critical if you want to control which tissues your genes of interest are delivered to. Although some serotypes can infect multiple tissues, by carefully selecting a tissue-specific promotor in your construct, you can maximise expression in your desired tissue and limit off-target effects. The VVC will work with you to ensure that the most appropriate serotype is selected for your specific requirements. Below is a list of capsids that the VVC currently has available, with additional capsids available on request.
Capsid | Tissues |
|---|---|
AAV1 | CNS, brain lung, retina, pancreas, liver, inner ear, skeletal muscle, smooth muscle, heart |
AAV2 | CNS, brain, liver, pancreas, kidney, retina, inner ear, testes, smooth muscle |
AAV5 | CNS, brain, smooth muscle, heart, lung, retina |
AAV6 | Smooth muscle, heart, lung, pancreas, adipose, liver |
AAV8 | CNS, brain, smooth muscle, retina, inner ear, liver, pancreas, heart, kidney, adipose |
AAV9 | CNS, skeletal muscle, smooth muscle, heart, lung, liver, pancreas, retina, inner ear, testes |
PHP.eB | CNS |
PHP.S | PNS |
AAV-DJ | Immortalised cell lines, liver |
MyoAAV 4A | Cardiac & skeletal muscle |
MG1.2 | Microglia |
TM6 | Microglia |
Additional capsids available on request.
Comparison to other viral vectors
Characteristic | Lentivirus | AAV | Adenovirus |
|---|---|---|---|
Genome | 9 kb (ssRNA) | 4.8 kb (ssDNA) | 36 kb (dsDNA) |
Packaging limit | 7.5 kb | 4.7 kb | 8 kb |
Expression | Stable | Transient or stable | Transient |
Integration | Integrating | Non-integrating | Non-integrating |
Transduction Efficiency | Moderate | Moderate | High |
Immunogenicity | Low | Very Low | High |
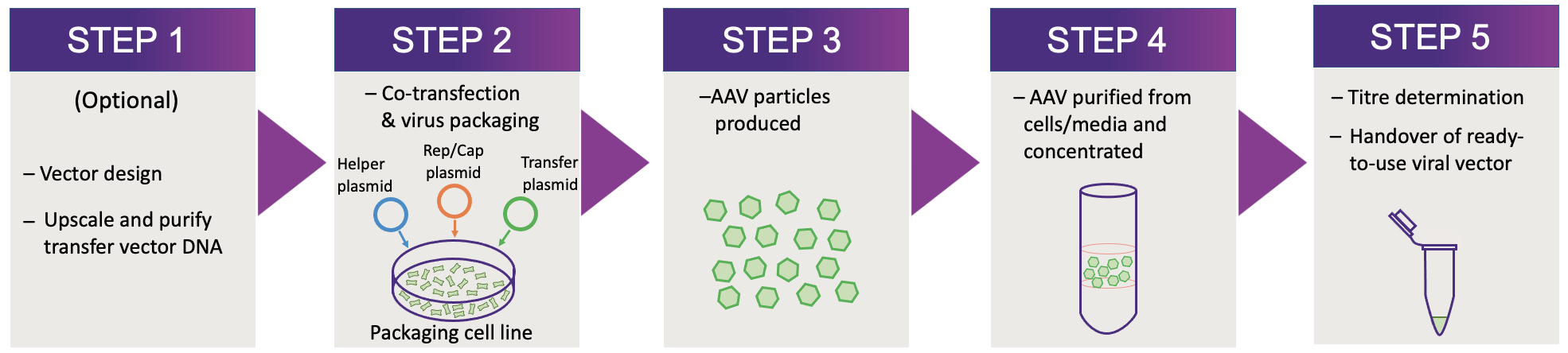
Workflow
The VVC uses optimised workflows and validated protocols to produce high-quality viral vectors. To get started, please contact us or submit a booking to assist with designing a vector that is right for you. Depending on the complexity of the transfer vector, we can either clone it in-house or outsource this to an external company. You can also choose to clone and supply the transfer vector yourself if you wish.
To produce AAVs, three plasmids are transfected into an AAV packaging cell line - pRep/Cap (containing viral replication and capsid genes), pHelper (containing Adenovirus helper packaging genes) and the transfer vector (containing a user-defined cassette). The AAV particles then begin to be produced using the packaging cell line's machinery.
After a few days, the AAV-containing cells and supernatant are collected and AAVs are then purified and concentrated into a small volume. Viral titre is determined by measuring the copy number of viral (or transgene) elements via qPCR (standard) or ddPCR (additional cost), with a final concentration given as viral genome copies (vgc) per mL. Upon completion, you will receive your viral vector along with any additional QC information that you requested (if applicable).